-
Faculty of Arts and HumanitiesDean's Office, Faculty of Arts and HumanitiesJakobi 2, r 116-121 51005 Tartu linn, Tartu linn, Tartumaa EST0Institute of History and ArchaeologyJakobi 2 51005 Tartu linn, Tartu linn, Tartumaa EST0Institute of Estonian and General LinguisticsJakobi 2, IV korrus 51005 Tartu linn, Tartu linn, Tartumaa EST0Institute of Philosophy and SemioticsJakobi 2, III korrus, ruumid 302-337 51005 Tartu linn, Tartu linn, Tartumaa EST0Institute of Cultural ResearchÜlikooli 16 51003 Tartu linn, Tartu linn, Tartumaa EST0Institute of Foreign Languages and CulturesLossi 3 51003 Tartu linn, Tartu linn, Tartumaa EST0School of Theology and Religious StudiesÜlikooli 18 50090 Tartu linn, Tartu linn, Tartumaa EST0Viljandi Culture AcademyPosti 1 71004 Viljandi linn, Viljandimaa EST0Professors emeriti, Faculty of Arts and Humanities0Associate Professors emeriti, Faculty of Arts and Humanities0Faculty of Social SciencesDean's Office, Faculty of Social SciencesLossi 36 51003 Tartu linn, Tartu linn, Tartumaa EST0Institute of EducationJakobi 5 51005 Tartu linn, Tartu linn, Tartumaa EST0Johan Skytte Institute of Political StudiesLossi 36, ruum 301 51003 Tartu linn, Tartu linn, Tartumaa EST0School of Economics and Business AdministrationNarva mnt 18 51009 Tartu linn, Tartu linn, Tartumaa EST0Institute of PsychologyNäituse 2 50409 Tartu linn, Tartu linn, Tartumaa EST0School of LawNäituse 20 - 324 50409 Tartu linn, Tartu linn, Tartumaa EST0Institute of Social StudiesLossi 36 51003 Tartu linn, Tartu linn, Tartumaa EST0Narva CollegeRaekoja plats 2 20307 Narva linn, Ida-Virumaa EST0Pärnu CollegeRingi 35 80012 Pärnu linn, Pärnu linn, Pärnumaa EST0Professors emeriti, Faculty of Social Sciences0Associate Professors emeriti, Faculty of Social Sciences0Faculty of MedicineDean's Office, Faculty of MedicineRavila 19 50411 Tartu linn, Tartu linn, Tartumaa ESTInstitute of Biomedicine and Translational MedicineBiomeedikum, Ravila 19 50411 Tartu linn, Tartu linn, Tartumaa ESTInstitute of PharmacyNooruse 1 50411 Tartu linn, Tartu linn, Tartumaa ESTInstitute of DentistryL. Puusepa 1a 50406 Tartu linn, Tartu linn, Tartumaa ESTInstitute of Clinical MedicineL. Puusepa 8 50406 Tartu linn, Tartu linn, Tartumaa ESTInstitute of Family Medicine and Public HealthRavila 19 50411 Tartu linn, Tartu linn, Tartumaa ESTInstitute of Sport Sciences and PhysiotherapyUjula 4 51008 Tartu linn, Tartu linn, Tartumaa ESTProfessors emeriti, Faculty of Medicine0Associate Professors emeriti, Faculty of Medicine0Faculty of Science and TechnologyDean's Office, Faculty of Science and TechnologyVanemuise 46 - 208 51003 Tartu linn, Tartu linn, Tartumaa ESTInstitute of Computer ScienceNarva mnt 18 51009 Tartu linn, Tartu linn, Tartumaa ESTInstitute of GenomicsRiia 23b/2 51010 Tartu linn, Tartu linn, Tartumaa ESTEstonian Marine Institute0Institute of PhysicsInstitute of ChemistryRavila 14a 50411 Tartu linn, Tartu linn, Tartumaa EST0Institute of Mathematics and StatisticsNarva mnt 18 51009 Tartu linn, Tartu linn, Tartumaa EST0Institute of Molecular and Cell BiologyRiia 23, 23b - 134 51010 Tartu linn, Tartu linn, Tartumaa ESTTartu ObservatoryObservatooriumi 1 61602 Tõravere alevik, Nõo vald, Tartumaa EST0Institute of TechnologyNooruse 1 50411 Tartu linn, Tartu linn, Tartumaa ESTInstitute of Ecology and Earth SciencesJ. Liivi tn 2 50409 Tartu linn, Tartu linn, Tartumaa ESTProfessors emeriti, Faculty of Science and Technology0Associate Professors emeriti, Faculty of Science and Technology0Institute of BioengineeringArea of Academic SecretaryHuman Resources OfficeUppsala 6, Lossi 36 51003 Tartu linn, Tartu linn, Tartumaa EST0Area of Head of FinanceFinance Office0Area of Director of AdministrationInformation Technology Office0Administrative OfficeÜlikooli 17 (III korrus) 51005 Tartu linn, Tartu linn, Tartumaa EST0Estates Office0Marketing and Communication OfficeÜlikooli 18, ruumid 102, 104, 209, 210 50090 Tartu linn, Tartu linn, Tartumaa EST0Area of RectorRector's Strategy OfficeInternal Audit OfficeArea of Vice Rector for Academic AffairsOffice of Academic AffairsUniversity of Tartu Youth AcademyUppsala 10 51003 Tartu linn, Tartu linn, Tartumaa EST0Student Union OfficeÜlikooli 18b 51005 Tartu linn, Tartu linn, Tartumaa EST0Centre for Learning and TeachingArea of Vice Rector for ResearchUniversity of Tartu LibraryW. Struve 1 50091 Tartu linn, Tartu linn, Tartumaa EST0Grant OfficeArea of Vice Rector for DevelopmentCentre for Entrepreneurship and InnovationNarva mnt 18 51009 Tartu linn, Tartu linn, Tartumaa EST0University of Tartu Natural History Museum and Botanical GardenVanemuise 46 51003 Tartu linn, Tartu linn, Tartumaa EST0International Cooperation and Protocol Office0University of Tartu MuseumLossi 25 51003 Tartu linn, Tartu linn, Tartumaa EST0
A new approach has the potential to double the efficiency of energy storage devices.

Scientists from the University of Tartu and the University of Copenhagen have reached a theoretical description of an ideal electrocatalysis process. If the process could be realized in practice, it would be possible to operate energy conversion and storage devices twice as efficiently, writes Novaator.
The world is seeking sustainable solutions to meet the growing energy demand. Recently, scientists from the University of Tartu and the University of Copenhagen proposed a new approach that allows overcoming long-standing limitations in oxygen electrocatalysis.

Catalysis occurs when some accelerating additive, called a catalyst, is added to a chemical reaction. For instance, certain natural proteins or enzymes can make substances react faster with each other. Electrocatalysis also involves an electrode, which is some kind of electrically conductive body. In electrocatalysis, substances start to react with each other on the surface of the electrode.
Oxygen electrocatalysis involves oxygen evolution and reduction reactions. These are extremely important in various electrochemical energy conversion and storage systems, such as water electrolysis, fuel cells, and metal-air batteries.
"These reactions are complex because they involve breaking and forming multiple chemical bonds, which usually have high activation energy. To accelerate these processes, an extremely active catalyst is needed, capable of efficiently lowering these energy barriers and facilitating reactions," said Nadežda Kongi, the leader of the inorganic functional materials research group (KongiLab) at the University of Tartu and an associate professor.
She noted that overcoming these limitations and accelerating the transition to a hydrogen economy requires a new paradigm for designing catalysts. "Our research group has discovered a way to bypass these theoretical limitations," she said.
In a recent article published in the Royal Society of Chemistry's journal Catalysis Science and Technology, the research group introduced an innovative approach: geometry-adaptive electrocatalysis. The approach uses catalysts whose shape changes during the reaction. In this way, the theoretical limitations that have hindered the development of oxygen electrocatalysis for decades can be overcome.
The new method allows bypassing the energy-intensive breaking and forming of chemical bonds in electrocatalysis and instead offers a shortcut (see the diagram below). "This concept could revolutionize the field of oxygen electrocatalysis," said the main author of the study, Ritums Cepitis, a fourth-year doctoral student at the University of Tartu's Institute of Chemistry.
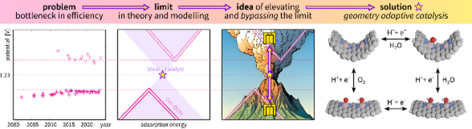
Adapting geometry during oxygen electrocatalysis causes catalysts to behave ideally and raises them to the peak of activity volcano.
Author: Cepitis et al. 2024/Catalysis Science & Technlogy
"Our model shows that ideal catalysis is within reach, and practically, it could potentially double the efficiency of energy conversion and storage technologies," added Vladislav Ivaništšev, who developed the idea together with Professor Jan Rossmeislig during a postdoctoral fellowship at the University of Copenhagen.
"Now our group in Tartu is ready to implement this approach. Laboratory work requires even more creativity than the modeling stage, but we are already seeing promising progress," said Kongi.
Read more similar news






